T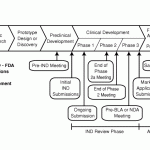 here are now quite a few different ways that myotonic dystrophy can be defeated. To the left is the pathway that the FDA requires and the most advanced pathways are still in animal studies. This is a nice article but technical.
here are now quite a few different ways that myotonic dystrophy can be defeated. To the left is the pathway that the FDA requires and the most advanced pathways are still in animal studies. This is a nice article but technical.
Therapeutics Development in Myotonic Dystrophy Type I
Abstract
Myotonic dystrophy (DM1), the most common adult muscular dystrophy, is a multi-system, autosomal dominant genetic disorder caused by an expanded CTG repeat that leads to nuclear retention of a mutant RNA and subsequent RNA toxicity. Significant insights into the molecular mechanisms of RNA toxicity have led to the surprising possibility that treating DM1 is a viable prospect. In this review, we briefly present the clinical picture in DM1, and describe how the research in understanding the pathogenesis of RNA toxicity in DM1 has led to targeted approaches to therapeutic development at various steps in the pathogenesis of the disease. We discuss the promise and current limitations of each with an emphasis on RNA-based therapeutics and small molecules. We conclude with a discussion of the unmet need for clinical tools and outcome measures that are essential prerequisites to proceed in evaluating these potential therapies in clinical trials.
Background and Clinical Relevance
Myotonic dystrophy type 1 (DM1) is a common and debilitating form of muscular dystrophy that preferentially affects adolescents and young adults. With a prevalence of ranging from 1:8000 (in Caucasians) to approximately 1:20000 (Asian, Japanese and African populations), it is the most common muscular dystrophy in adults and the second most common muscular dystrophy overall 1,2. In certain populations, such as the Saguenay region of Quebec, prevalence can approach 1 in 500 due to founder effects and population isolation 3. The predominant feature of the disease is weakness, but it is a multisystem disorder with other serious and life-threatening manifestations. In its most common form, onset of symptoms occurs during adolescence or in the second decade of life, and affected individuals have a significantly shortened lifespan of 48–55 years 4–6. However, a severe congenital carries a neonatal mortality rate of 16% 7. A second related form of the disease, myotonic dystrophy type 2 (DM2) has similar disease manifestations, although they are generally less severe and usually of later onset 8.
DM1 is an autosomal dominant CTG triplet repeat disorder with variable expression. In successive generations, the phenomenon of genetic anticipation is often observed and is associated with instability of the repeat sequence. Individuals with five to 37 repeats are asymptomatic and not at risk for having affected offspring. A repeat length of 38–49 is considered a “premutation”- that is, the carrier of this repeat expansion is unaffected, but due to anticipation, offspring can have higher repeat numbers and manifest aspects of the disease. A repeat length of greater than 50 is considered a full mutation, and although disease severity is variable, all individuals with repeat lengths of this size or greater are affected to some extent.9 Skeletal muscle pathology is the most characteristic feature of DM1. Impaired muscle relaxation from myotonia can lead to stiffness and cramping, especially in the distal muscles of the hands, but it is rarely a significant patient complaint. Muscle weakness and wasting start distally and progress proximally over time and often lead to severe disability as the disease progresses. Respiratory muscle involvement is common, and respiratory failure either from the primary muscles process or from cardiopulmonary involvement is a significant contributor to patient mortality 10.
A less well defined but important disease feature is its effect on the central nervous system and cognition, which can be manifested by low IQ, dementia, and apathy 11–13. The emotional and behavioral issues often predate other systemic symptoms 14. Recent work has demonstrated global deficits with neuropsychologic testing as well as radiographic changes in the brains of affected individuals, including an increased white matter lesion burden, decreased gray matter mass (especially in the hippocampal and thalamic regions), and hypometabolism in the frontal lobes 15,16. In addition to the effects on the central nervous system, 90% of patients will develop cardiac abnormalities at some point, the most common of which is varying degrees of heart block. These and other cardiac conduction abnormalities can lead to the most feared complication of DM1, sudden cardiac death. Frequent monitoring and early recognition of conduction defects is critical to management and may lead to preemptive implantable cardioverter defibrillator (ICD) and/or pacemaker placement 17–20. One of the most common patient complaints is fatigue and hypersomnolence, which are multifactorial in myotonic dystrophy. Abnormal sleep architecture, hypercapnia, and sleep apnea are common causes, and they should be monitored for and addressed appropriately 21.
Congenital myotonic dystrophy (CDM) is almost exclusively maternally inherited, although rare cases of paternal inheritance have been described 22. Maternal repeat lengths greater than 300 are associated with a higher risk of CDM, but genetic anticipation makes true prediction of risk nearly impossible 23. Affected pregnancies often come to attention secondary to polyhydramnios or diminished fetal movements, and prematurity is common. Profound neontal hypotonia is often present, and severe diaphragmatic and intercostal muscle weakness can lead to absent or minimal respiratory effort at birth. If a neonate requires prolonged artificial ventilation at birth, the mortality rate is greater than 25% 24. Affected children have other associated dysmorphic features which may include arthrogryposis, clubfoot, and abnormalities in facial features. Early systemic effects can include swallowing difficulties, mental retardation, and cardiomyopathy 25. The characteristic myotonia is absent in neonates, so a diagnosis is often based on family history or clinical suspicion. Survivors may strengthen somewhat, but they ultimately develop a progressive myopathy similar to the more common adolescent-onset form of the disease.
DM2, also known as proximal myotonic myopathy (PROMM) was first described in the mid-1990’s and was genetically mapped to human chromosome 3q in 1998 26,27. To date, no congenital form has been described. Although also a repeat disorder, DM2 is caused by an expansion of CCTG repeats in intron1 of of the zinc finger gene, ZNF9 28. Unlike DM1, where the distal musculature displays early involvement and weakness, in DM2 the hip girdle is affected first. This pattern of weakness onset can help clinically distinguish the two entities 29.
To date, the only therapeutic interventions for patients with DM are targeted towards symptom management. However, significant advances in understanding the complex pathophysiology have led to numerous approaches to targeting disease mechanisms that demonstrate great promise in pre-clinical studies. This review will highlight some of these. The goal is to give a firm understanding of the current direction of translational approaches to treating DM1 with an eye to the future of clinical trials for potential therapeutics.
Molecular Pathogenesis and Description of Model
DM1 is caused by an expanded CTG repeat in the 3′ untranslated region (UTR) of the dystrophia myotonica protein kinase gene (DMPK) on chromosome 19 30–32. The expanded repeat is transcribed into DMPK mRNA, but it does not affect DMPK protein structure. Rather, this expansion leads to production of a mutant DMPK mRNA which forms aggregates in affected nuclei (reviewed in 33). Key studies have shown that the relative haploinsufficiency of the DMPK protein is not responsible for disease phenotype 34. With the discovery that the DM2 mutation is a (CCTG)n repeat within a gene called ZNF9 on chromosome 3 and that this leads to RNA aggregates similar to those seen in DM1, it has been posited that the myotonic dystrophies may be the first examples of disorders caused by RNA toxicity.
How does an accumulation of aberrant RNA lead to disease? It is proposed that many clinical manifestations of the disease are caused by alterations in the levels and activity of specific RNA-binding proteins involved in RNA splicing, and that changes in levels and stability of these proteins then lead to aberrant splicing of many downstream RNA targets (reviewed in 35, 36). In general, the resultant spliceopathy leads to a splicing pattern more consistent with fetal expression than normal adult splicing patterns. Specifically, a protein known as Muscleblind-like protein 1 (MBNL1) has been found to co-localize with mutant DMPK mRNA foci, leading to the hypothesis that MBNL1 is sequestered by the RNA foci resulting in a loss of function that ultimately affects downstream targets 37,38. Recent analysis has suggested that at least 200 targets are mispliced in a mouse model of DM1 and that >80% of these misplicing events are likely due to functional MBNL1 loss 39. In support of this, genetic deletion of MBNL1 leads to a subset of the phenotypes seen in DM1, namely cataracts, splicing alterations, myotonia, and changes in muscle histology 40. Interestingly, MBNL1 is sequestered with RNA foci in DM2 as well, and many aspects of the spliceopathy seen in DM1 are also found in DM2 41.
In addition to MBNL1 sequestration, it has been demonstrated that levels of another RNA-binding protein, CUG-binding protein 1 (CUGBP1) are increased in affected tissues. This is thought to be mediated by Protein Kinase C (PKC) phosphorylation and resultant stabilization of CUGBP1, although the details of this mechanism are poorly understood 42. CUGBP1, like MBNL1, has important roles in RNA splicing and is thought to be antagonistic to MBNL1 for many of the splicing defects observed in DM1. Thus, the increase in CUGBP1 could synergize with the functional loss of MBNL1. CUGBP1 also has additional roles in RNA stability and translation 43–46. Unlike the situation for DM1, CUGBP1 levels do not appear to be changed in DM2, and it has been suggested that this may account for some of the clinical differences seen in DM1 and DM2 patients 47.
Missplicing of certain downstream targets is then considered directly responsible for at least some of the observed phenotypes of DM1, and new targets are continually being discovered. For example, it has been elegantly shown that mis-splicing of the muscle-specific chloride channel ClC1 is responsible for the myotonia observed in DM1 models and patients. Aberrant splicing of the ClC-1 pre-mRNA leads to inclusion of exon 7a into the mature mRNA (a pattern more consistent with embryonic ClC-1 expression). Exon 7a inclusion ultimately results in a premature stop codon, resulting in rapid decay of the mis-spliced transcript 48,49. Other splicing abnormalities may be responsible for insulin resistance (aberrant splicing of insulin receptor), some of the cardiac phenotypes (cTNT), and likely some of the central nervous system cognitive effects 50–53. A summary of the steps in the pathogenesis of DM1 is shown in Figure 1, and the instances in which molecular or symptomatic therapy have been attempted are noted.

Figure 1
Many mouse (and non-murine) models of DM1 have been developed, but all have certain shortcomings. Importantly, no single mouse model exists that perfectly mimics the human disease, either in expression profile, molecular dysfunction, or resultant phenotype and disease progression. Transgenic mouse models expressing expanded repeats under muscle-specific promoters have been developed, but the disease phenotype is limited to the muscle pathology 34,54. Thus, in these models, therapeutics could not be assessed for their effect on other systemic manifestations of DM1. However, these same models have proven extremely useful for studying muscle-specific effects and potential interventions, as detailed below.
Our lab developed a mouse model of DM1 using a doxycycline inducible, human DMPK promoter with the goal of expressing the toxic RNA in the same tissues as in DM1. Importantly, induced overexpression of the toxic RNA leads to many of the phenotypes observed in DM1 patients, including progressive cardiac conduction deficits, myotonia, weakness, splicing abnormalities, histopathologic changes, and increased CUGBP1 levels. However, despite mimicking the disease phenotype so well, these mice do not display obvious RNA foci and thus do not sequester MBNL1 in large nuclear inclusions. Interestingly, shutting down production of the toxic RNA in this model leads to reversion of many of the phenotypes, providing an important proof of principle regarding reversibility of disease through a therapeutic approach based on targeting the mutant DMPK transcript for destruction. Interestingly, this model does not involve an expanded repeat, but rather overexpression of a (CTG)5-repeat containing 3′UTR 55. It remains to be seen whether or not this will serve as an adequate model for development of therapeutics.
General concepts in therapeutic design strategy are detailed in Figure 2. Targeting the earliest stage of aberrant pathophysiology is often the most difficult and technically challenging approach (in this case targeting DNA repeat expansion), although it has the potential for the greatest therapeutic benefit since all downstream effects are then modified. As one targets further down the cascade, potential therapies may be easier to design and implement, however, only a subset of pathology would be treated. For example, targeting splicing abnormalities in the chloride channel mRNAs in DM1 patients may be more feasible than targeting DNA repeat expansions. The resultant effect, however, would address only the myotonia in skeletal muscle, and other systemic complications would still be present. Thus, an ideal therapy would need to strike a pragmatic balance to allow for substantial benefit, relative ease of technical approach, and minimal effect on unrelated cellular mechanisms. Theoretically, and in practice, each of the abnormal steps in the pathogenesis of DM1 could be targeted for interventional modification. Below we will discuss the varied approaches as well as their potential applications and limitations to human disease.

Figure 2
Translational Approaches to disease modification
Targeting repeat expansion and instability in DNA
To date, this option remains a theoretical possibility only. Repeat expansion occurs in both somatic and germ line tissues and occurs in tissue-specific, developmentally-regulated, and cell-specific manners. The complex mechanisms controlling this process remain poorly understood at this point. Recent work to elucidate mechanisms of repeat instability, as well as work targeted towards understanding repeat contractions in particular, may ultimate lead to novel therapeutic approaches to DM1 and other repeat disorders (reviewed in 56). In the future, targeting of mismatch repair mechanisms, methylation pathways, and tissue and developmentally-regulated DNA replication machinery may prove to be valuable in controlling repeat instability and reducing the burden of anticipation and triplet repeat expansion. In addition, small molecules that target the repeat sequence itself and thus interfere with normal cellular processing also have promise 57, 58.
Targeting the toxic RNA
Thus far, targeting the mutant RNA itself appears to hold some of the greatest prospect for therapeutic intervention in DM1. In general, this approach uses one of two methods, ribozymes and antisense oligonucleotides. Ribozymes are RNA molecules, either native or engineered, that adopt a tertiary structure and function as catalysts for a reaction (and therefore, as enzymes). Thus, they can cleave other RNAs and ultimately lead to degradation of their targets or lead to splicing events that replace target RNA with embedded sequences 59. Ribozymes were first applied to DM1 when a group studying DMPK repeats used a tetrahymena group 1 intron ribozyme to cleave, in vitro, a DMPK RNA sequence containing the endogenous 12 repeats and replace them with a 5 repeat sequence contained in the engineered ribozyme. Subsequent experiments by the same group indicated that similar splicing and replacement could be achieved in human cultured fibroblasts 60,61. These studies were performed on fibroblasts containing a normal repeat length. As such, whether this technique could be applicable in an expanded repeat model and how mutant RNA could be easily distinguished from wild-type transcript by the ribozyme was not addressed.
In 2003, Puymirat et al. published an important study utilizing a hammerhead ribozyme (named so for its characteristic tertiary structure) to target mutant RNA. This particular study capitalized on the fact that mutant DMPK is retained in the nuclei to help ensure that the engineered ribozyme did not target wild-type transcript. Despite this, both mutant and wild-type transcripts were effectively reduced (63% mutant transcript, 50% WT transcript). They also observed a concomitant decline in nuclear foci and a partial restoration of normal splicing of the insulin receptor 62. Before ribozyme technology can be effectively used for gene therapy, both the issues of specificity and delivery method must be adequately addressed.
Aside from ribozymes, there are other promising approaches to targeting mutant RNA transcript, including the use of antisense oligonucleotides (AON). It is important to note that the term “antisense oligonucleotide” actually encompasses at least four chemically different molecule types, some of which will be described below. At its most basic, these are short, single-strand stretches of nucleic acids that hybridize via Watson-Crick base-pairing to complementary cellular “sense” mRNAs. This binding then inhibits gene expression and, in some cases, ultimately can target the mRNA for degradation. The chemistry of these various AONs is complex, however, it is worth mentioning that at least two forms of AONs are currently being employed for possible therapeutic interventions in DM1 and other muscular dystrophies. The first is the 2′-O-methyl modified AONs (MOE), and the other is the phosphorodiamidate morpholino antisense molecules (PMO or morpholino). Of note, morpholinos differ from other antisense strategies in that they provide inhibition via steric hindrance, rather than by any targeted degradation63. They offer a promising antisense technology due to their increased stability, improved permeability, and high target specificity over traditional AONs 64.
In one of the first studies to use antisense technology in DM, Furling et al. demonstrated that transduction of DM1 cells in culture with an adenoviral vector expressing an antisense RNA to CUG repeats was capable of reducing mutant DMPK mRNA. In addition, CUGBP1 expression levels were reduced in treated cells, and the ability to respond to insulin was improved 65. Certainly, the main limitation of this study was its restriction to cell culture, but the results were quite promising. The use of adenoviral vectors provides an advantage over straightforward AON technology in that the virus, once introduced, can continuously provide the therapeutic molecule over time, prolonging the potential therapeutic dosing window.
In 2009, Mulders et al described a (CAG)7 AON of the MOE subtype that selectively targets the DMPK RNA. They tested this molecule both by delivering it directly to cells in culture and by local injection and electroporation into mouse tissues in two different murine DM1 models. The group observed degradation of mutant DMPK RNA in certain muscles (up to 50%), as well as diminished RNP foci and a reversal of selected splicing deficits 66. This data provides important proof of principle regarding the potential for use of AONs in DM1 models. However, this strategy had important limitations. Only local injection was performed, and certainly systemic methods will need to be employed in patients. In addition, degradation of the wild-type transcript with a concurrent decrease in protein levels was observed, although to a much lesser extent, and it is not yet clear whether this can have a detrimental effect on the tissue.
More recently, an exciting report has emerged detailing the use of a morpholino designed to disrupt the interaction between MBNL1 and the mutant RNA transcript 67. Details of this will be described below, but it deserves mention that the group that pursued this approach noticed both their intended effect of disruption of RNA/protein interaction and a significant degradation of the primary mutant RNA transcript itself. It remains to be determined how much of the observed effect of the morpholino’s administration was due to the former mechanism of action and how much was due to the latter.
Targeting the structural RNA hairpin
Besides targeting nascent RNA, other groups have attempted to target the particular hairpin structure that is formed by the expanded CUG repeats. In particular, these long hairpin structures have been shown to be substrates for Dicer, a ribonuclease whose main function in the RNA interference pathway is to recognize double-stranded RNA duplexes and induce cleavage of these structures into shorter, 21-nt duplexes. These duplexes are then incorporated into the RNA-induced silencing complex (RISC), and as such, function to guide substrate selection for degradation (reviewed in 68). Krol et al have demonstrated that this mechanism is active in cells faced with expanded (CUG)n repeats. Furthermore, introduction of a synthetic oligoribonucleotide (CUG)7 into DM1 patient fibroblasts led to a selective reduction in transcripts containing very long repeats 69. This has lent support to the hypothesis that using siRNAs (small interfering RNAs) to target the mutant RNA in DM1 would then tag the RNA for destruction via the Dicer pathway. In 2005, Langlois et al demonstrated that introduction of a lentivirus-delivered short hairpin RNA (shRNA, which is processed in the cell to 21-nt siRNAs) into DM1 myoblasts led to a preferential reduction in mutant nuclear RNA (cytoplasmic wild-type transcript was degraded as well with a concordant ~55% decrease in DMPK protein levels) 70. More work is needed before this approach can be considered to be viable in vivo, as delivery and selectivity remain active and important issues.
Targeting Protein-RNA binding
As mentioned previously, it is thought that accumulation of mutant RNA causes sequestration of MBNL1, thus altering functional levels of this protein and disrupting downstream splicing events. Thus, a reasonable therapeutic approach would be to attempt to disrupt RNA-MBNL1 binding, freeing the protein to perform its native function. Several attempts have been made to accomplish just this, using small molecules as well as engineered oligonucleotides.
A recent series of papers has detailed the approach by Disney et al to develop modular ligands with various properties that bind to (C/CUG)n repeats and subsequently displace MBNL1 binding. A peptide backbone with kanamycin A modules was found to have high affinity for the target sequence, and various modifications were made to the basic structure to increase its binding capacity 71,72. Small ligands offer an intriguing option for disease modification given their permeability and tight binding affinities. The in vivo applications of these particular ligands remains uninvestigated 73,74.
Another small molecule offering great promise for potential disease modification in DM1 was described by Warf et al in 2009. In this study, the investigators screened a library of small molecules known to bind to tertiary nucleic acids structures. The screen relied on the use of a gel-shift assay that would allow the researchers to assess whether the small molecule could disrupt a preformed MBNL1-(CUG)4 complex. They discovered that pentamidine, an antifungal drug currently in use to treat Pneumocystis carinii infection in immunocompromised patients, was capable of doing just that. Using a HeLA cell culture model with expanded repeats (CUG)960, the group was able to determine that pentamidine treatment could rescue alternative splicing of the insulin receptor and cardiac troponin T (cTNT) transcripts to levels observed in HeLa cells without CUG repeats. The pentamidine treatment did not affect splicing outcomes on a global level and appeared to be specific to MBNL1-regulated transcripts. In addition, MBNL1 colocalization with RNA foci was significantly diminished in the cell culture model. The authors then assessed the outcome of pentamidine treatment in a mouse DM1 model which expressed 250 CUG repeats under the control of a muscle-specific promoter. The drug was delivered in an intraperitoneal fashion, and splicing assays were performed after mouse sacrifice. Partial reversal of the aberrant splicing was observed, and drug toxicity appeared to be the limiting factor in furthering the observed effect 75. Pentamidine is highly toxic and has a very narrow therapeutic window, and thus its use at the doses required to mediate meaningful phenotypic change is unlikely to be feasible. The authors suggest that modification to pentamidine’s structure in an attempt to retain function and decrease toxicity is warranted, and such experiments are likely underway.
In a recent and very exciting publication, Wheeler et al have described the design and use of a morpholino AON to target and disrupt expanded CUG repeat/protein complexes. In vitro, this morpholino termed CAG25 was able to invade and stably bind CUG hairpins. In addition, the group demonstrated that this binding was able to not only block new MBNL1/CUG complex formation but also disrupt protein/RNA complexes that had already formed via MBNL1 displacement. Moreover, they then tested the morpholino’s biochemical effect in a mouse model of DM1 that expresses expanded repeats in a skeletal-muscle specific pattern. Intramuscular injection and electroporation of the morpholino resulted in some striking results, including a decrease in RNA foci with concurrent dispersement of MBNL1, near normalization of splicing abnormalities that persisted for at least 14 weeks after AON treatment, and marked reduction of myotonia secondary to restoration of proper CLC1 function. Of note, the morpholino had no effect when it was tested in an mbnl1-deficient mouse which manifests many of the same splicing abnormalities as DM1 models. This was to be expected if the mechanism of action of the morpholino was as proposed, working via an effect on expanded repeats which are not present in the mbnl1 knockout model. As mentioned earlier in this review, application of this morpholino to the mouse model also resulted in an unpredicted 50% reduction in mutant RNA via an unknown post-transcriptional mechanism. The authors propose that this reduction in RNA burden is not solely responsible for the improved function, because the residual RNA levels are above the threshold needed to cause disease 67.
While this approach is promising, the ever-present issue of delivery in a clinical setting is still relevant 64. However, two recent reports from the Duchenne Muscular Dystrophy (DMD) field offer an exciting glimpse into the real possibilities of antisense therapies for DM1. Unlike the triplet-repeat mediated mechanism of disease in DM1, DMD is caused by point or frame-shift mutations in the dystrophin gene on the X chromosome. The majority of these mutations lead to the absence of functional protein (in contrast to Becker muscular dystrophy in which a truncated dystrophin results in a less severe phenotype). Although hundreds of mutations have been identified that lead to DMD, there are common mutations that offer high-yield targets for therapeutic interventions. One potential disease-modifying approach in DMD involves using antisense technology to bind to the mutated region of the RNA transcript. This binding then prohibits this region of the RNA from being incorporated into the final mRNA and restores a normal reading frame, a process known as “exon skipping”. Although the resultant protein is shorter than its wild-type counterpart, the majority of protein function is maintained 63,76.
This technique has been validated via local injection/delivery studies, similar to what has recently been reported by Wheeler et al using the morpholino in DM1 models (reviewed in 77). However, this approach has moved into phase II studies for DMD, and two recent reports suggest that intravenous or subcutaneous systemic delivery of these molecules appears to be well-tolerated and safe. Both groups employed AON technology targeted towards exon skipping of exon 51, a region that is responsible for roughly 13% of DMD cases. The pharma-supported phase two studies are not designed or powered for efficacy, but preliminary results indicate that dystrophin is being produced in muscle fibers in a dose-dependent fashion. In at least one of the studies, there was a suggestion of potential clinical efficacy as well as measured by the 6-minute walk test, but larger controlled trials are necessary before interpreting this data 78,79.
Altering levels of RNA binding proteins
Based on the models that have been been proposed for the pathophysiology of DM1, it would stand to reason that increasing levels of MBNL1 or decreasing levels of CUGBP1 in affected vertebrates could abrogate some of the phenotypes observed. Again, it is worth referring to the concept in figure 2. Certainly targeting the pathway at this stage could prove to be beneficial, however the therapeutic impact is presumably lower given the complexity of the cellular processes this far removed from the pathophysiology of the expanded repeats. Two important murine studies have lent some credence to this approach and will be briefly reviewed here.
As noted previously, mbnl1 knockout mice develop some, though not all, features of DM1, including myotonia, abnormal skeletal muscle histopathology, cataracts, and splicing abnormalities. This is consistent with the model that has been presented for the role of MBNL1 sequestration in disease pathology 40. In 2006, Kanadia et al proposed the natural extension of this model by asking whether increasing MBNL1 levels in a murine model for DM1 would lessen the observed disease phenotype. To address this, they developed an adenoviral vector which expressed a common skeletal muscle isoform of MBNL1 and injected this vector into the tibialis anterior muscle of HSALR mice (mice carrying an expanded CUG repeat under the actin promoter). They then demonstrated that overexpression of MBNL1 occurred in treated muscles and that this overexpression led to a reduction in myotonia and a restoration of normal chloride channel splicing (as well as other splicing deficits). The abnormal muscle histopathology was unchanged 80. Despite this latter observation, it remains an intriguing possibility that exogenous delivery of MBNL1 may lead to modification of some disease manifestations in affected individuals. Adenoviral vectors, though promising, still must overcome many technical hurdles before their role in disease treatment becomes commonplace.
Recently, overexpression of CUGBP1 in an inducible, muscle-specific manner was found to recapitulate many of the features of DM1 81. In a complementary study, Cooper and colleagues asked whether prevention of PKC-mediated phosphorylation of CUGBP1 in a mouse model expressing mutant RNA in an inducible heart-specific manner could reduce cardiac disease burden. Their model, EpA960, had been previously characterized and shown to develop conduction deficits, RNA foci with MBNL1 colocalization, increased CUGBP1 levels secondary to hyperphosphorylation, and increased mortality. Treatment of these mice with a PKC inhibitor, Ro-31-8220, significantly improved mortality (from 80% mock-treated to 20% treated). Concomitantly, CUGBP1 was not hyperphosphorylated, and protein levels were not elevated. Cardiac conduction and contractility were also improved in the treated mice. In addition, the switch to embryonic splicing patterns was reversed for targets of CUGBP1 but not for transcripts known to be modified by MBNL1. Importantly, use of Ro-31-8220 did not improve mortality in a second mouse model in which CUGBP1 was overexpressed in the heart, arguing against nonspecific and pleiotropic effects of the compound 82. The authors do not discuss the applicability of the compound to human disease states, but the study suggests a role for modification of CUGBP1 levels in targeting cardiac specific disease manifestations (the role in other tissues was not examined).
Alteration of downstream splicing targets
Although it is not clear whether all of the phenotypes in DM1 are caused by aberrant splicing, certainly evidence has accumulated that some of the key features are. Thus, reversal of splicing abnormalities directly using antisense technology or other targeting techniques is a viable potential approach to disease modification. Although many of the interventions discussed above have the secondary effect of modifiying splicing patterns, few papers have focused on the targeting of this specific step. One of the limitations of targeting the pathway this far down is its limited return. In other words, antisense technology designed to reverse the chloride channel missplicing may ultimately lead to less myotonia in affected individuals, but the cardiac, GI, CNS, and dystrophic features would still be present. Targeting multiple misspliced transcripts may ultimately be a viable therapeutic approach, but much greater study and refinement is needed. There may be some advantage to designing therapy aimed at missplicing of those transcripts that result in significant patient impairment. In addition, attempting to modify those targets that lead to the most serious of the disease manifestations, such as cardiac conduction deficits, could have great clinical benefit.
To this end, Wheeler et al described the use of another morpholino AON to target the missplicing of the chloride channel that causes the characteristic myotonia in DM1. The AON was designed to induce suppression of exon 7a inclusion during the splicing process and thus lead to full-length protein production. Indeed, delivery of the antisense molecule in two mouse models resulted in splicing normalization, increased CLCl full length mRNA and protein levels, improved chloride channel function and abrogation of myotonia 83. This study provides important proof of principle that targeting such downstream events can modify the disease process.
Symptom Management
There are numerous therapeutic approaches in DM1 that do not rely on disease modification but rather target solely symptom management. The use of mexelitine, which modulates sodium channels and thus lessens myotonia, and CNS stimulants to address fatigue are two such examples that are routinely applied in practice 84. Other interventions have been met with less success. A 12-week trial of dehydroepiandrosterone (DHEA) administration in DM1 patients was not efficacious in terms of the primary outcome of manual muscle testing (MMT) score from baseline to week 12. The drug did not provide any benefit in any of the secondary outcome measures either, including changes in quantitative muscle testing and timed functional testing, respiratory and cardiac function, and quality of life 85. It is not clear whether the length of time of drug administration was sufficient given the chosen outcome measures.
Early data had suggested that delivery of exogenous insulin-like growth factor (IGF1) could improve muscle strength and function in adult DM1 patients 86, presumably owing to its anabolic effect on muscle. However, delivery of this therapy required twice daily subcutaneous injections, and therapeutic effect was small. A larger phase II study using a recombinant form of IGF1 called IPLEX with a longer half-life and improved ease of delivery was undertaken in the pharmaceutical sector, and the clinical field eagerly awaited the results. Unfortunately, in June 2009, the parent company Insmed announced that the drug did not improve muscle function, strength or endurance, and further analysis of the compound’s use in DM1 was halted. IPLEX remains in study for other neuromuscular disorders.
Conclusions and Future Directions
Much progress has been made regarding the development of molecular therapeutics for myotonic dystrophy, and there is great promise inherent in many of these approaches. However, before we are able to move these therapies forward into clinical trials, we must first identify the appropriate outcome measures to be used as markers for therapeutic efficacy. This requires adequate longitudinal studies of disease progression. In addition, it will require the identification of molecular disease biomarkers that can be used to follow the extent of disease modification with therapeutic intervention. Clearly, if the outcome measures chosen in the clinical trial design do not adequately reflect changes in disease state, then these new therapies may be deemed to be ineffective despite real benefit. New initiatives are being developed to identify appropriate biomarkers and to uncover which outcome measures are most reliable and meaningful for the purpose of clinical trials.
Research in DM1 has opened up new frontiers in medical research. It is now almost twenty years since the discovery of the DM1 mutation. Its discovery was at the earliest stages of a whole new field in human genetics, namely the study of repeat expansion mutations. Definition of the pathogenesis of DM1 led to the novel concept of RNA toxicity, a concept which is now implicated in several other disorders. More recently, translational research has clearly suggested the possibility that DM1 may be potentially reversible. Ultimately, the unique properties of pathogenesis of this disease has led to the development of several promising therapeutic strategies that make it plausible to envision therapy designed to halt progression or even reverse damage induced by the toxic RNA.
Acknowledgments
Acknowledgements of grants:
Work in the Mahadevan lab is supported by NIAMS (RO1AR052771 and RO1AR045992) and the Muscular Dystrophy Association. Erin Pennock Foff is supported by the NINDS Research Education Program R25 NS065733
List of abbreviations used in the manuscript
- DM1
- myotonic dystrophy type 1
- DM2
- myotonic dystrophy type 2
- ICD
- implantable cardioverter defibrillator
- CDM
- congenital myotonic dystrophy
- PROMM
- proximal myotonic myopathy
- UTR
- untranslated region
- AON
- antisense oligonucleotides
- MOE
- 2′-O-methyl modified antisense oligonucleotide
- PMO
- phosphorodiamidate morpholino antisense molecules
- RISC
- RNA-induced silencing complex
- siRNA
- small interfering RNA
- shRNA
- short hairpin RNA
- DMD
- Duchenne muscular dystrophy
- DHEA
- dehydroepiandrosterone
- MMT
- manual muscle testing

