Many couples in which one partner has myotonic dystrophy want to have children. There is a study that looks at pre-Implant diagnosis here the threat of the disease is eliminated. This is a good route for many couples. If universal education were available it may be possible to greatly reduce this disease.
Preimplant-Study-Pregnancy-Myotonic-DystrophyCategory Archives: Potential Treatments Thereputics
FDA approved Chemotherapy Drug Might Treat Myotonic Dystrophy
In theory this might be a treatment for myotonic dystrophy. This has not been tried in humans and would be highly risky but for people near end of life this may be a vector for them and their doctors to consider.
Researchers have previously identified what they think is the cause of the disease.In Myotonic Dystrophy the repeat expansion mutation is made into RNA but it does not get out into the cytoplasm. It remains trapped in the nucleus where it sticks to various proteins and appears as spots or foci that can be observed down the microscope. Because these proteins are stuck to the repeat RNA they cannot perform their normal functions correctly within the cell.
Researchers have found that to make progress with this disease, they need to “unstick” the proteins. This drug appears to do this in mice and cells.
Previously to the publication of this article there was no even theoretical treatment available. There are several drugs in development but this takes years of development. For those near end of life with this disease there is now a potential treatment. A copy of the article is here. This is something you may want to discuss with your medical team. Its untried and potentially risky with side effects. More information will be available shortly.
Please note the study is very technical. We are not recommending this to anyone but bringing all the current information to your attention
New Company Atricode Formed to Pursue Myotonic Dystrophy Drug Development (MDI16)
2012 USC Ideas Empowered Program Grant: $90,000
Atricode is developing treatments for rare diseases. The lead indication, Myotonic Dystrophy Type 1 (DM1,) is a devastating genetic multisystem disorder with no available treatment or cure. This team identified highly potent and selective small molecule leads that rescue DM1 pathology in patient myoblasts and in DM1 mouse models. The team joined forces with experienced entrepreneurs to form a start-up company that moves the drug candidates towards the clinic. The drug candidate could be the first therapy to treat this devastating disorder and the Ideas Empowered funds are critical for the selection of the lead candidate for clinical development and to support fundraising efforts.
Potential Drug Pathways May 2011
T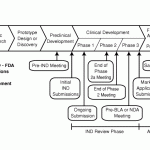 here are now quite a few different ways that myotonic dystrophy can be defeated. To the left is the pathway that the FDA requires and the most advanced pathways are still in animal studies. This is a nice article but technical.
here are now quite a few different ways that myotonic dystrophy can be defeated. To the left is the pathway that the FDA requires and the most advanced pathways are still in animal studies. This is a nice article but technical.
Therapeutics Development in Myotonic Dystrophy Type I
Abstract
Myotonic dystrophy (DM1), the most common adult muscular dystrophy, is a multi-system, autosomal dominant genetic disorder caused by an expanded CTG repeat that leads to nuclear retention of a mutant RNA and subsequent RNA toxicity. Significant insights into the molecular mechanisms of RNA toxicity have led to the surprising possibility that treating DM1 is a viable prospect. In this review, we briefly present the clinical picture in DM1, and describe how the research in understanding the pathogenesis of RNA toxicity in DM1 has led to targeted approaches to therapeutic development at various steps in the pathogenesis of the disease. We discuss the promise and current limitations of each with an emphasis on RNA-based therapeutics and small molecules. We conclude with a discussion of the unmet need for clinical tools and outcome measures that are essential prerequisites to proceed in evaluating these potential therapies in clinical trials.



