A new study by several major researchers in the myotonic dystrophy field had a very interesting study that identified a FDA approved drug that may help with myotonic dystrophy. However, as usual, more research is needed to validate the approach. The study identified and validated a cell based assay screening tool that enabled the researchers to look at a number of drugs. The most promising drugs were colchicine, thiocolchcine,suprafenacine, amsacrine, azathioprine, The researchers decided to concentrate on was Colchicine an already approved FDA drug for gout and familial Mediterranean fever (FMF),
“We primarily focused on colchicine because it is an inexpensive, FDA-approved, natural therapeutic that is generally well tolerated and is currently used in the clinic to treat gout and familial mediterranean fever (FMF)“‘
The next step the researchers took was to use colchicine in mice that had been altered to have myotonic dystrophy. The drug was injected into these mice. The researchers found that the amount of mutant RNA was decreased in the muscle cells.
“Collectively, these data demonstrate that microtuble inhibition in vivo leads to a selective reduction in expanded CUG RNA levels without broadly affecting the transcriptome”
Next the researchers tested colchicine in patient cells with myotonic dystrophy. They selected patients cells with a repeat count of 1900-3000 repeats. The results were positive
“we observed significant rescue of missplicing”
OVERALL DISCUSSION
The researchers established a cell line that enabled them to screen a large number of compounds that might help to reduce DM1 in theory. They found a list of candidates and then selected colchicine as a drug to model
“We then validated the use of a microtubule inhibitor in the HSA DM1 mouse model and in DM1 patient cells with colchicine an FDA approve natural microtubule inhibitor currently used in the clinic. Our results provide proof of principle for the identification of compounds and cellular targets selectively modulater r(CUG)exp levels in DM1 using cell-based screening.
Our Observation of a partial rescue in DM1 relevant missplicing in multiple models warrants further evaluation of colchicine. The study is NOT sufficient to address the therapeutic efficacy of colchicine or of general microtuble inhibition in the treatment of DM1, It is important to determine if there is a positive trade off between the therapeutic efficacy in reducing DM1 symptoms in relation to known toxicity from microtuble inhibition. As an example, and although very rare, myopathy has been reported in some individuals with compromised renal function who had been treated chronically with colchicine for gout. Future Long term treatment in DM1 animal models such as HSA at clinical doses to evaluate the reversal of DM1 phenotypes are a [prerequisite to determine if any clinical studies are warranted”
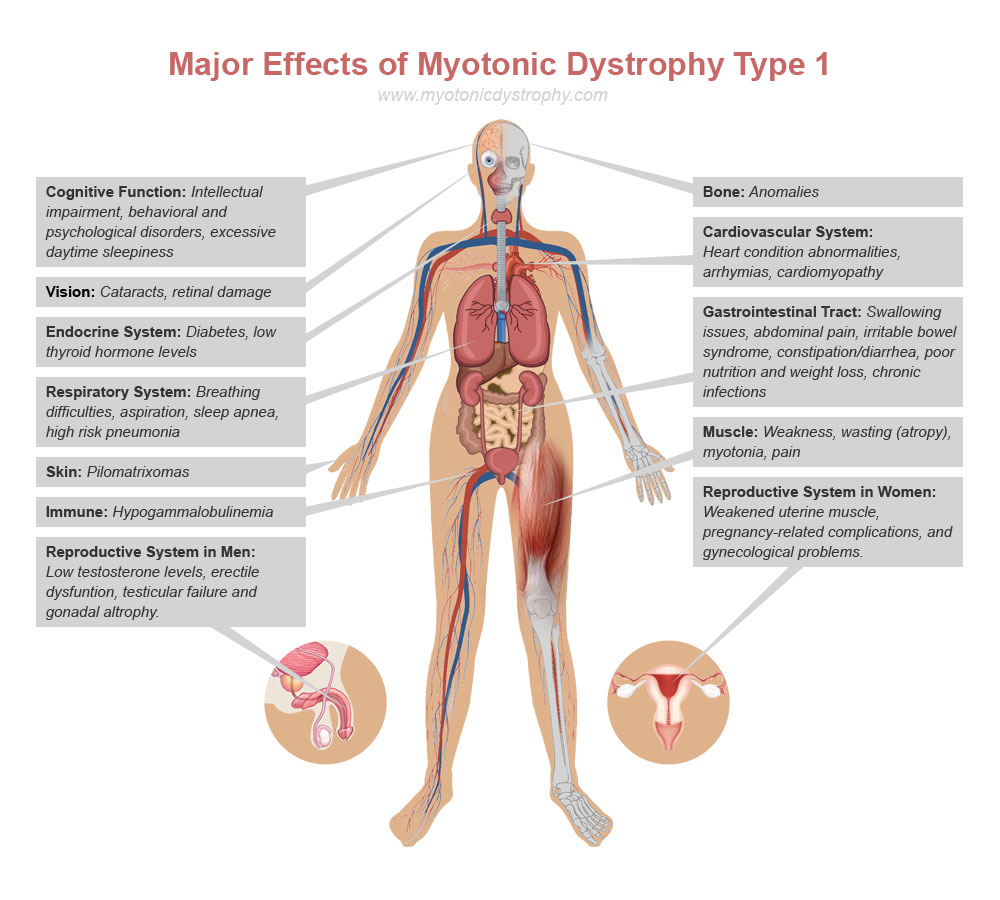
Overall this is a promising approach that needs more study. However, for those in the end stages of Myotonic dytrophy disease this may be something to discuss with your medical staff.
The transcriptome can be seen as a subset of the proteome, that is, the entire set of proteins expressed by a genome.. However, the analysis of relative mRNA expression levels can be complicated by the fact that relatively small changes in mRNA expression can produce large changes in the total amount of the corresponding protein present in the cell.
colchicine-Study
PDF Embedder requires a url attribute