The Muscular Dystrophy Association of the USA (MDA) announced its winter research grants. 13.6 million was awarded including two grants for Myotonic Dystrophy research. One grant to doctor Disney will explore the possible therapeutic effects of small molecule approach for DM2. This would be a strong complement to the DM1 research that is ongoing in his lab.
The other grant to Dr. Mockton is to research the effect of Triplet expansion and stability on the likely course of the Myotonic Dystrophy Disease.
MDA is spending 4.6 of its research grant budget this winter on Myotonic Dystrophy Research.
DM — Matthew Disney, Ph.D.
Matthew Disney, associate professor of chemistry at The Scripps Research Institute in Jupiter, Fla., was awarded an MDA research grant totaling $362,724 over a period of three years to test the ability of compounds he has developed to target the toxic RNA in myotonic dystrophy type 2 (MMD2, also known as DM2).
MMD2 is caused by an abnormally expanded section of DNA on chromosome 3, which leads cells to make an abnormally long segment of RNA from it. This expanded RNA folds into a structure that traps certain cell proteins, preventing them from doing their normal jobs.
Disney’s research group has designed small molecules that bind specifically to this expanded RNA. If these, or molecules like them, can reduce the amount of protein the RNA can trap, they may be useful as therapy for MMD2. Disney will be testing these molecules for that ability, and also determining the three-dimensional structure of the MMD2 RNA.
“If we are successful in these endeavors we will be able to better understand how DM2 RNA binds to and inactivates proteins,” he says. That should provide the foundation for development of better and more specific molecules that can be tested as drugs in people with MMD2.
“This is very much a developing field, and a lot of work is needed to establish if small molecules can be designed to target RNA and have therapeutic utility,” Disney says. “These studies are critically important because biologists are doing such a phenomenal job in identifying the biochemical mechanisms of disease. The major question is whether we can leverage this mechanistic information into small-molecule therapies.”
Funding for this MDA grant began Feb. 1, 2013.
MMD — Darren Monckton, Ph.D.
Darren Monckton, professor of human genetics at the University of Glasgow in Scotland, was awarded an MDA research grant totaling $273,892 over a period of two years to develop new diagnostic tests for myotonic muscular dystrophy type 1 (MMD1, also known as DM1)
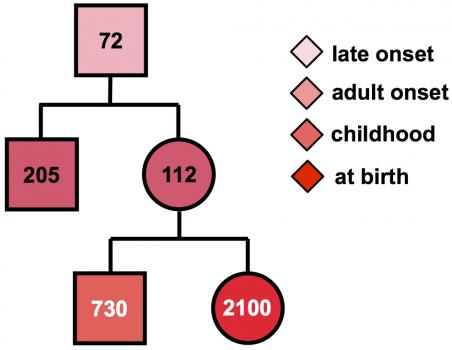
MMD1 is caused by expansion of part of the DNA code in the DMPK gene. Normally, this gene has a small number of repeats of the DNA “letters” CTG. But in people with MMD1, the number of repeated CTG units is 50 or more, and can be over 1000. Furthermore, the number of repeated units in the genes can grow over the lifetime and may differ between different cells.
“In the standard diagnostic test, this genetic instability is ignored,” Monckton says, “and only an average number of repeats is measured.” But the variation may contain important clues to when the disease is likely to begin, and so may provide valuable information to families and individuals with the MMD1 repeat about the future course of disease.
“Using specialized research techniques, we can measure the number of CTG repeats in many cells, then use a computer model to calculate the number of CTGs the patient had at birth,” Monckton notes. “This number much more accurately predicts when symptoms are likely to start. In addition, the CTGs are sometimes interrupted by other groups of three letters, such as CCG, usually leading to milder symptoms,” and later onset in future generations. “Unfortunately, the current genetic test does not take account of age-dependent changes in the number of repeats or detect the presence of these interruptions.”
Monckton will collaborate with genetic testing laboratories to develop new diagnostic tests that will allow the detection of both these important features. In addition to benefits for individual families, the new tests should help make the interpretation of clinical trial results more accurate, since any effects of treatment can be measured against the severity of the genetic mutation.
Funding for this MDA grant began Feb. 1, 2013.