Because of the disease characteristics in DM1 and DM2, appropriate molecular testing and reporting is very important for the optimal counseling in myotonic dystrophy. Here, we describe best practice guidelinesfor clinical molecular genetic analysis and reporting in DM1 and DM2, including presymptomatic and prenatal testing.
Category Archives: Genetic Information
Cuyamycin Molecule looks promising for Myotonic Dystrophy Treatment
A new study published shows that a small molecule (Potential new treatment) disrupted the long CTG repeats but left short repeats mostly alone.This was tested in mice that had myotonic dystrophy and seemed to work well. Here is a piece form the journal about the impact of this study
Significance
Development of small-molecule lead medicines that potently and specifically modulate RNA function is challenging. We designed a small molecule that cleaves r(CUG)exp, the RNA repeat expansion that causes myotonic dystrophy type 1. In cells and in an animal model, the small-molecule cleaver specifically recognizes the 3-dimensional structure of r(CUG)exp, cleaving it more selectively among transcripts containing short, nonpathogenic r(CUG) repeats than an oligonucleotide that recognizes RNA sequence via Watson-Crick base pairing. The small molecule broadly relieves disease-associated phenotype in a mouse model. Thus, small molecules that recognize and cleave RNA structures should be
MDA Issues Winter Research Grants
The Muscular Dystrophy Association of the USA (MDA) announced its winter research grants. 13.6 million was awarded including two grants for Myotonic Dystrophy research. One grant to doctor Disney will explore the possible therapeutic effects of small molecule approach for DM2. This would be a strong complement to the DM1 research that is ongoing in his lab.
The other grant to Dr. Mockton is to research the effect of Triplet expansion and stability on the likely course of the Myotonic Dystrophy Disease.
MDA is spending 4.6 of its research grant budget this winter on Myotonic Dystrophy Research.
Myotonic Dysrophy Affects over 10 Members of One family
How muscular dystrophy cruelly affected 10 of my close family, by MP Dave Anderson
By Dave Anderson, Mp For Blaydon, Tyne And Wear
UPDATED: 17:33 EST, 10 October 2009
 Fight: Dave Anderson is calling for more resources
Fight: Dave Anderson is calling for more resources
My brother Bill played an immense role in my life. For one thing, he gave me my love for music. In 1962 he was bringing home Marvin Gaye records while our friends were listening to The Bachelors. And we were there together, in July 1969, when the Rolling Stones took over Hyde Park in front of thousands.
He taught me to drive and he gave me my love of the outdoor life. He convinced me to go into mining and told me about an opportunity to gain an international scholarship – both of which put me where I am today.
When, in 2001, I was made vice-president of Unison, at that time the biggest union in the country – where I stayed until being elected as a Member of Parliament four years later – my wife organised a surprise party for me. We had a great time, but the joy was muted as Bill was no longer around to share my pride.
Genetic Information
|
There are two types of Myotonic Dystrophy
DM1 is known as Type 1
DM2 is known as Type 2, and also known as PROMM or “Proximal Myotonic Myopathy“ DM1 is caused by an expanded repeat of CTG on Chromosome 19
DM2 is caused by an expanded quadruplet repeat of CTG on Chromosome 3 Differences in DM1 and DM2
DM2 does not seem to cause the severe congenital form when the mother that has the disease has an infant. Also the apathetic personality traits with DM1 do not seem to be associated with DM2. People with DM2 will have more weakness in the trunk of the body (proximal) versus DM1 will have more weakness in the distal parts of the body like legs and arms. The incidence of DM2 is unknown. There is a genetic test for DM2 just available from Athena Diagnostics. Estimation is that the incidence is less than 2% (QUEST Feb 2002) but it may be much higher.
A deficient gene causes DM1. This is an alteration in the myotonin protein kinase gene, specifically in the APOC2 locus located on the proximal long arm of chromosome 19. DM is an autosomal dominant condition; this means that only one copy of the gene with a genetic alteration is necessary for DM to occur. The genetic alteration found in the myotonin protein kinase is a repeating sequence of three specific nucleotides (these are the ‘building blocks’ which make up DNA). Normally in gene 19 the sequence of cytosine-thymidine-guanine (CTG) repeats itself from 5-37 times in a row, however in an altered DM gene this CTG sequence repeats 50+ times and in some cases it can exceed 2000. The number of repeats effects when the age of onset occurs and the severity of DM. The table below shows how the CTG repeat length/numbers effects the clinical symptoms
|
|||||||||||||||||||
| Table 1: Correlation of CTG repeat numbers with clinical symptoms | |||||||||||||||||||
|
|||||||||||||||||||
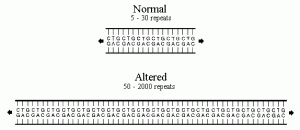 |
The numbers of CTG repeats are stable from generation to generation when they are in the normal 5-30 repeats. The number of CTG repeats may change in number from generation to generation when they are in the range of 50+. The number of repeats will rarely decrease in number from parent to child. Figure 1 shows the difference between the normal and the altered CTG repeats |
||||||||||||||||||
|
Some authorities maintain that the correlation between the number of repeats and the severity of the illness is not absolute. A key factor they maintain is the age when the first symptoms appear. The earlier the symptoms appear the more likely that the disease may have a more severe manifestation. The affected mother has a 50% chance of transmitting the mutation to each child. The underlying Myotonic Dystrophy that the mother has is inherited as a “autosomal dominant trait” However, there is some thought that the mother has a “maternal factor” that passes through the placenta and thus is the cause of fetal muscle maturation arrest. (Farkas-Bargeton). There have a a very few documented cases of paternal (father’s) transmission of the congenital form. Click here for more info. Congenital Myotonic Dystrophy is related and linked very strongly to Myotonic Dystrophy. In virtually all cases the mother must be affected and have Myotonic Dystrophy. Myotonic Dystrophy is a genetic disease that is more progressive between generations. This is called anticipation; that means that each son or daughter that is affected may have the disease slightly worse. The CMD condition is potentially the worst outcome of this disease. Myotonic Dystrophy has the anticipation or amplification factor. Each succeeding generation may have the disease slightly worse. The number of repeats of the CTG sequence gets longer. The onset of Myotonic Dystrophy gets earlier as a general rule as the number of repeats increases. In this family of disorders the number of repeats tend to increase with succeeding generations. Again, this is known as “genetic anticipation” There also has been a report from England that there can be regression (shrinkage) as the MD allele is passed on to successive generations; this is mostly seen with paternal (through the father which is rare) transmission. The is also some reports of regression to a repeat number within the normal range Myotonic and CMD belong to a growing family of disorders where the genetic mutation involves unstable Trinucleotide repeats (C_G) These letters correspond to amino acids which form the basis of DNA. More information on Triplet Repeat Disorders or Triplet Repeat Information. A few related disorders: Fragile X Syndrome CGG |
|||||||||||||||||||
| CTG Repeat Numbers | |||||||||||||||||||
|
5 repeat and 16-30 repeat alleles have been shown to share the same linkage disequalibrium as DM. It is proposed that the 5 repeat allele expands to alleles in the 16-30 repeat range which then form a pool for recurrent DM mutations. In the absence of a high new mutation rate, this theory explains why myotonic dystrophy is maintained in the population and not lost via the congenital form. |
|||||||||||||||||||
 |
|||||||||||||||||||
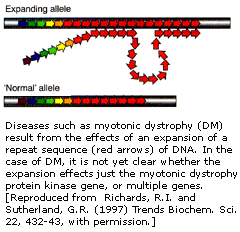
| Diagram of How the disease may affect transcription
|
|||||||||||||||||||
Click below for a great booklet on Whether to test for Myotonic Dystrophy |
|||||||||||||||||||
| myotonic dystrophy an informed choice of testing | |||||||||||||||||||

