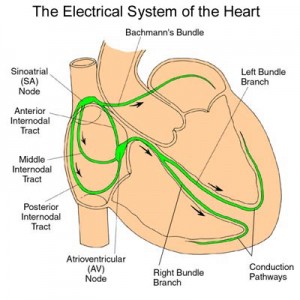
New information on a heart problem that is present in many of the adult patients with myotonic dystrophy – atrial flutter. This is the heart beating too fast in the upper chambers of the heart. This new information from France gives an over view and possible treatments for this symptom. Sorry the whole article is not available for free!
Tag Archives: cardiac
How does Myotonic Dystrophy affect the brain and higher level functioning
A recent study gave more information on how Myotonic Dystrophy affects the brain. This information is important as treatments are being developed and there are many questions on whether this will help with brain related issues. The study below gives information on white matter and gray matter in the brain that were obtained with MRI studies. The Gray matter seems to give some indication of how a patient may be affected with The various symptoms of myotonic dystrophy. Here is a summary of the study. Click Below to get the full study in PDF
Patients even with short CTG repeats with Myotonic Dystrophy may have cardiac issues
A new case study has found that some patients with short DM repeats less than 100 who may not have symptoms of the disease may in fact be troubled with cardiac issues. Here is a full text of the study’s conclusions
This case shows that MD1 with <100 CTG repeats may
exclusively manifest cardiologically, that family screening
for MD1 is important even in asymptomatic patients, and
that MD1 may initially manifest with atypical clinical features.
Muscle biopsy in MD1 may be misleading and may
indicate glycogenosis. Close cardiac follow-up is important
if MD1 manifests cardiologically to prevent syncope or SCD.
Patients even with short repeats may have cardiac issues may 2015
Heart Review Myotonic Dystrophy
Editors note: This article was written in 2002 approximately 10 years ago. Its a review but more current information must be reviewed as well
MYOTONIC DYSTROPHY AND THE HEART
Myotonic dystrophy (dystrophia myotonica, DM) is the most frequently inherited neuromuscular disease of adult life. DM is a multisystem disease with major cardiac involvement. Core features of myotonic dystrophy are myotonia, muscle weakness, cataract, and cardiac conduction abnormalities. Classical DM (first described by Steinert and called Steinert’s disease or DM1) has been identified as an autosomal dominant disorder associated with the presence of an abnormal expansion of a CTG trinucleotide repeat on chromosome 19q13.3 (the DM 1 locus). A similar but less common disorder was later described as proximal myotonic myopathy, caused by alterations on a different gene on chromosome 3q21 (the DM2 locus). This article will mainly focus on DM1. It will provide an insight into the epidemiology and genetic alterations of the disease and provide up-to-date information on postmortem and clinical findings and on diagnostic and therapeutic options in patients presenting cardiac involvement.
EPIDEMIOLOGY AND CLASSIFICATION OF DM1
The incidence of DM1 is estimated to be 1 in 8000 births and its worldwide prevalence ranges from 2.1 to 14.3/100 000 inhabitants.1 Based on the age of onset and on its clinical features, DM1 can be divided into three forms: congenital, classical, and minimal, which may occur in the same kindred.
Pacemakers extend life of some Myotonic Dystrophy Patients
An invasive strategy, based on prophylactic permanent pacing, is associated with longer survival for patients with myotonic dystrophy type 1.
 Karim Wahbi, MD, of Pitié-Saltpêtière Hospital in Paris, and colleagues conducted a retrospective study of 914 consecutive patients (>18 years) with genetically confirmed myotonic dystrophy type 1 who were admitted to the hospital from 2000–2009. Of the 486 patients whose electrocardiogram showed a PR interval >200 ms or a QRS duration >100ms, or both, 70.2% underwent an invasive treatment strategy based on systematic electrophysiological studies and prophylactic permanent pacing and 29.8% underwent a noninvasive strategy.
Karim Wahbi, MD, of Pitié-Saltpêtière Hospital in Paris, and colleagues conducted a retrospective study of 914 consecutive patients (>18 years) with genetically confirmed myotonic dystrophy type 1 who were admitted to the hospital from 2000–2009. Of the 486 patients whose electrocardiogram showed a PR interval >200 ms or a QRS duration >100ms, or both, 70.2% underwent an invasive treatment strategy based on systematic electrophysiological studies and prophylactic permanent pacing and 29.8% underwent a noninvasive strategy.
During a median of 7.4 years of follow-up, the researchers found that 50 patients in the invasive strategy group and 30 in the noninvasive strategy group died (hazard ratio [HR], 0.74; P=0.19), corresponding to an overall nine-year survival of 74.4%. Adjusting for between-group differences in baseline characteristics, the invasive strategy was associated with significantly longer survival, with adjusted HRs ranging from 0.47–0.61. The survival difference was mainly due to a reduced incidence of sudden death, which occurred in 10 patients in the invasive strategy group vs. 16 patients in the noninvasive strategy group (HRs ranging from 0.24–0.28).
“Among patients with myotonic dystrophy type 1, an invasive strategy was associated with a higher rate of nine-year survival than a noninvasive strategy,” the authors write.
One author disclosed financial ties to the medical device industry; one author disclosed financial ties to Genzyme.
Abstract
Full Text (subscription or payment may be required)